What Type Of Covalent Bond Is The Longest
Both carbons are sp 3-hybridized meaning that both have four bonds arranged with tetrahedral geometryThe carbon-carbon bond with a bond length of 154 Å is formed by overlap of one sp 3 orbital from each of the carbons while the six carbon-hydrogen bonds are formed from. Living systems cannot directly utilize light energy but can through a complicated series of reactions convert it into C-C bond energy that can be released by glycolysis and other metabolic processes.

Bonding By Analogy Dog Bone Bonds Covalent Bonding Bond Metallic Bonding
Values are given for typical oxidation number and coordination.

What type of covalent bond is the longest. Elastomer any rubbery material composed of long chainlike molecules or polymers that are capable of recovering their original shape after being stretched to great extentshence the name elastomer from elastic polymerUnder normal conditions the long molecules making up an elastomeric material are irregularly coiled. Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. New Movie Releases This Weekend.
This lets us find the most appropriate writer for any type of assignment. With our money back guarantee our customers have the right to request and get a refund at any stage of their order in case something goes wrong. The structure of an ideal infinitely long single-walled carbon nanotube is that of a regular hexagonal lattice drawn on an infinite cylindrical surface whose vertices are the positions of the carbon atoms.
What type of reaction is represented by the following equation. Fluorine F chlorine Cl bromine Br iodine I and astatine At. The energy for this comes from the first phase of the photosynthetic process.
Of the various types of radiation gamma rays have the highest frequency. The largest database 1 of organic compounds lists about 10 million substances which include compounds originating from living organisms and those synthesized by chemists. In the modern IUPAC nomenclature this group is known as group 17.
Electromagnetic spectrum ranges from radiation with the highest frequency shortest wavelength to lowest frequency longest wavelength. Covalent radius Half of the distance between two atoms within a single covalent bond. The number of potential organic compounds has been estimated 2 at 10 60 an astronomically high number.
In the ethane molecule the bonding picture according to valence orbital theory is very similar to that of methane. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to. The halogens ˈ h æ l ə dʒ ə n ˈ h eɪ--l oʊ--ˌ dʒ ɛ n are a group in the periodic table consisting of five or six chemically related elements.
Since the length of the carbon-carbon bonds is fairly fixed there are constraints on the diameter of the cylinder and the arrangement of the atoms on it. Typically a nonmetal bonded to a nonmetal is a covalent. The artificially created element 117 tennessine Ts may also be a halogen.
Structure of single-walled tubes. Take A Sneak Peak At The Movies Coming Out This Week 812 Best Reactions to Movies Out Now In Theaters. Bond-line notation shows selected atoms as their chemical symbols while depicting some carbon atoms as corners between lines and omitting hydrogen atoms that are assumed to be in the structure.
Academiaedu is a platform for academics to share research papers. Bond-line notation is predominantly used in organic chemistry chemistry associated with living things. With the application of force however the molecules straighten.

Iupac Nomenclature Organic Chemistry For Naming Compounds Chemistry Organicchemistry Orgo N Organic Chemistry Study Chemistry Notes Organic Chemistry Notes

Alphabetical Priority Of Prefixes Chemistry Organic Chemistry Prefixes

The Bond S Name James Name Pleased To What Bond Name S The James Are You Alright Bames Nond S Having A Stronk Call A Bondulance Funny Memes Funny Quotes Bond
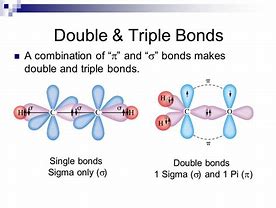
Of Single Double And Triple Covalent Bonds Which Is The Longest Which Is The Strongest Socratic

4 4 Characteristics Of Covalent Bonds The Basics Of General Organic And Biological Chemistry

Which Hydrogen Halide Has The Strongest Bond Bond Length Bond Chemistry

Organic Chemistry Educational Infographics Newman Projections Organic Chemistry Chemistry Educational Infographic

Naming Hydrocarbons Study Chemistry Chemistry Worksheets Chemistry Lessons

Organic Chemistry Science Educational School Posters Organic Chemistry Chemistry Classroom Organic Chemistry Study

Bond Length And Bond Strength Chemistry Steps

Converting Fischer Projection To Bond Line Structure Using The R And S And Swap Method Organic Chemistry Study Organic Chemistry Chemistry Lessons

Rules For Naming Alkanes Organicchemistry Orgo Ochem Mcat Chemistry Lessons Chemistry Classroom Organic Chemistry
Which Of The Following Has The Longest Bond Length C O N O C C Or C N Quora

Types Of Chemical Bonds Ionic Bond Covalent Bonds Coordinate Bonds Bas Covalent Bonding Ionic Bonding Chemical Bond

Difference Between Valence Electrons And Free Electrons Definition Occurrence Examples Effect On The Oxid Electrons Oxidation State Electron Configuration

Naming Alkenes Chemistry Physical And Chemical Properties Structural Formula

Triglyceride Easy Science Easy Science Triglycerides Fatty Acids

Organic Chemistry Educational Infographics Newman Projections Organic Chemistry Chemistry Educational Infographic

Pin By Cmlurrr On Studying School Organization Notes Notes Inspiration Study Notes