What Are The Two Types Of Covalent Bonds How Are They Different From Each Other
Binary Covalent Compounds Between Two Nonmetals. Each hydrogen atom lies on a line between two oxygen atoms and forms a covalent bond to one oxygen bond length.

Covalent Bond An Overview Sciencedirect Topics
It requires 8 electrons because that is the amount of electrons needed to fill a s- and p- orbital electron configuration.

What are the two types of covalent bonds how are they different from each other. A covalent bond between atoms is formed when they share one or more pairs of electrons among each other. Two p-orbitals remain unused on each sp hybridized atom and these overlap to give two pi-bonds following the formation of a sigma bond a triple bond as. It is a bonding between atoms within a molecule and forms the strongest bonds anywhere.
These types of bonds in chemical bonding are formed from the loss gain or sharing of electrons between two atomsmolecules. For Covalent bonds atoms tend to share their electrons with each other to satisfy the Octet Rule. The nucleus consists of protons and neutrons of different numbers in different elements.
Two nonmetals combine to form a covalent or molecular compound ie one that is held together by covalent bonds which result from the sharing of electrons. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities and is the primary interaction occurring in ionic compoundsIt is one of the main types of bonding along with covalent bonding and metallic bondingIons are atoms or groups of atoms with an electrostatic charge. In many cases two elements can combine in several different ways to make completely different compounds.
Covalent bonds are chemical bonds between two non-metal atoms. Also known as a. 100 Å and a hydrogen bond to the other hydrogen bond length.
Ionic Bonding Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another. Finally in the case of carbon atoms with only two bonding partners only two hybrid orbitals are needed for the sigma bonds and these sp hybrid orbitals are directed 180º from each other. The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing losing or gaining electrons--to become stable.
The tetrahedral shape of an individual water molecule is projected out into the surrounding crystal lattice. A molecule is two or more atoms bonded together to form a single chemical entity. Each atom carries a certain number of electrons that orbit around the nucleus.
Covalent bonds are the most common and most important kind of bonding.

Covalent Bond Definition Types And Examples
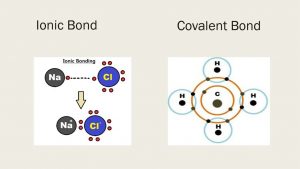
Chemical Bonds Ionic Bonds Properties Types Of Covalent Bonds Science Online

Single Covalent Bond Definition And Examples
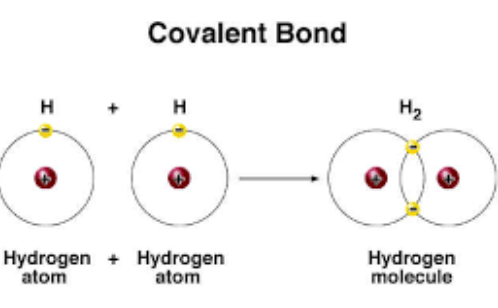
What Are The Types Of Covalent Bonds Science Online
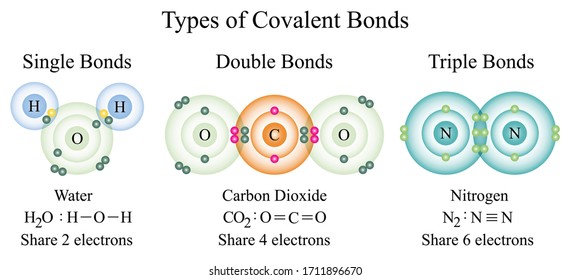
Covalent Bond Images Stock Photos Vectors Shutterstock
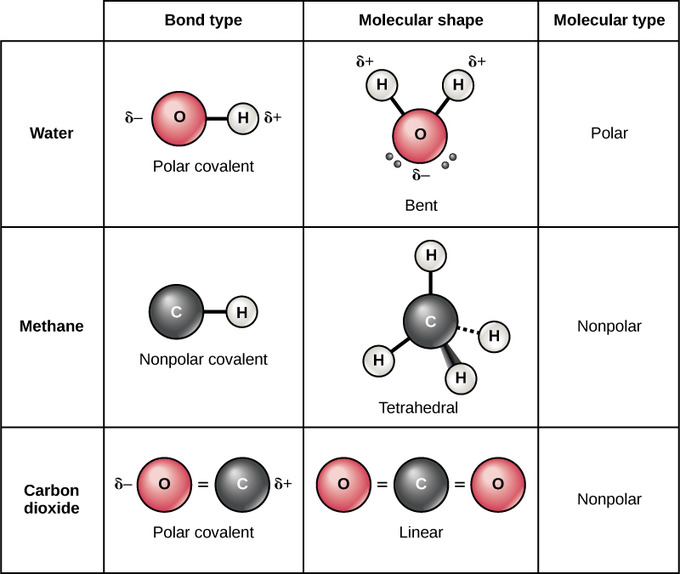
2 1i Covalent Bonds And Other Bonds And Interactions Biology Libretexts

Covalent Bond An Overview Sciencedirect Topics
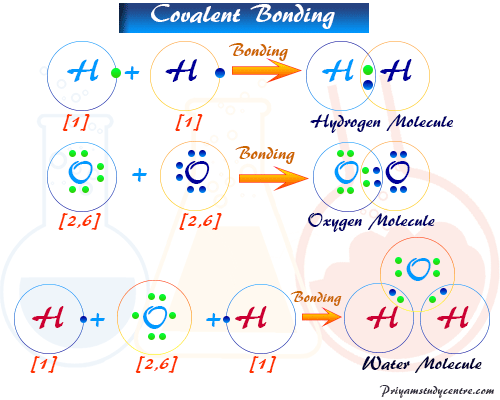
Covalent Bond Types Definition Properties Examples

Covalent Bond Class 11 Ch 4 Chemical Bonding And Molecular Structure
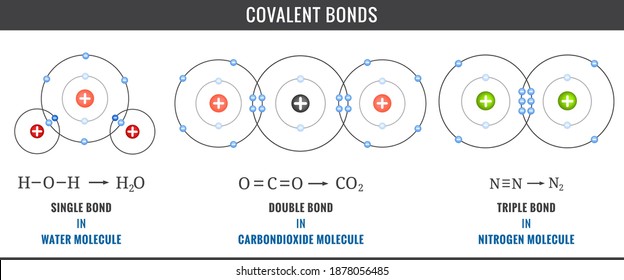
Covalent Bond Images Stock Photos Vectors Shutterstock
What Does A Covalent Bond Mean Quora

Covalent Bonding Biology Definition Role Expii

Covalent Bond Definition Types And Examples

What Are The Types Of Covalent Bonds Science Online
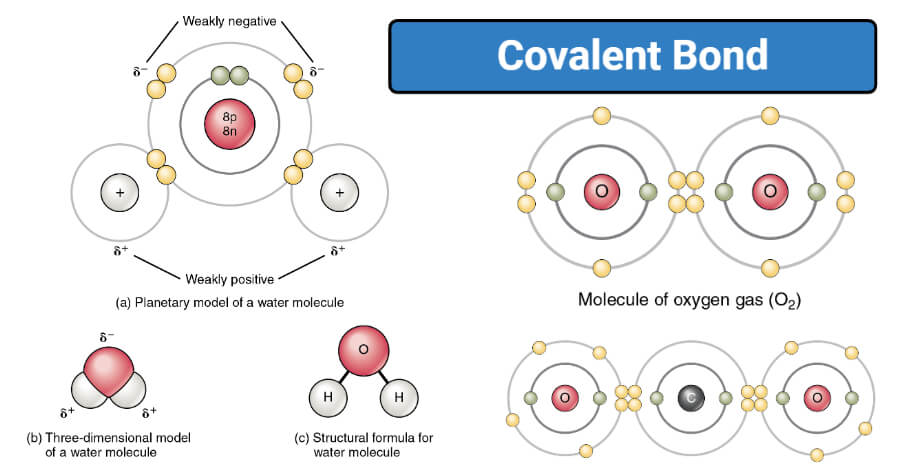
Covalent Bond Definition Properties Types Formation Examples

Covalent Bond Definition Types And Examples

Covalent Bond Definition Properties Examples Facts Britannica
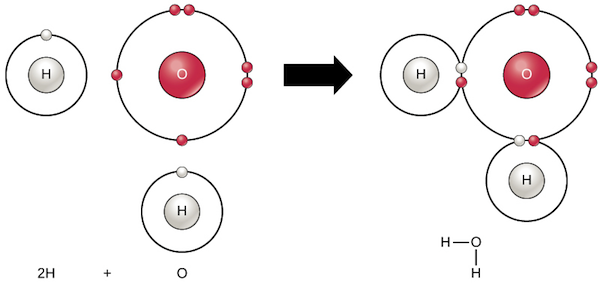
Chemical Bonds Chemistry Of Life Biology Article Khan Academy

Covalent Bond Biology Dictionary