What Are Ionic And Molecular Compounds Give Examples Class 9
Because each such clone is derived from a. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compoundsThe bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bondsThe strength of chemical bonds varies considerably.

How To Name Ionic And Covalent Compounds Chemistrynotes Com
Antibody secreted by a hybridoma clone.

What are ionic and molecular compounds give examples class 9. What is an Ionic Bond. The reverse is true of elimination reactions iethe number of σ-bonds in the substrate decreases and new π-bonds are often formedSubstitution reactions as the name implies are characterized by replacement of an atom or group Y by another atom or group Z. A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration.
The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond. In an addition reaction the number of σ-bonds in the substrate molecule increases usually at the expense of one or more π-bonds. Numerically the same as the relative molecular mass of a molecule expressed in daltons.
For example a protein of relative molecular mass 20000 has a molecular weight of 20000. There are primarily three ways in which two atoms combine. Understand Covalent Bonding with Properties Types - Polar Non-Polar bonds Difference between Covalent and Ionic Bonds Examples.
The solid consists of discrete chemical species held together by intermolecular forces that are electrostatic or Coulombic in nature. This behavior is most obvious for an ionic solid such as NaCl where the positively charged Na ions are attracted to the negatively charged Cl- ions. Covalent bonds are formed by equal sharing of electrons.
Group of atoms joined together by covalent bonds. In ionic and molecular solids there are no chemical bonds between the molecules atoms or ions. There are strong bonds or primary bonds such as.

Difference Between Empirical And Molecular Formula Infographic Chemistry Basics Chemistry Study Guide Chemistry Education

Elements Compounds Ionic Molecular Pure Substances Can Be Divided Into Elements And Compounds Compounds Are Molecular Pure Products Examples Of Mixtures

Naming Covalent Compounds Nomenclature Rules

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora

What Are Ionic And Molecular Compounds Give Exmples

Chemistry Lesson Identifying Ionic Vs Molecular Compounds Youtube

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

5 1 Ionic And Molecular Compounds Introductory Chemistry

Ionic Bond Examples Biology Dictionary
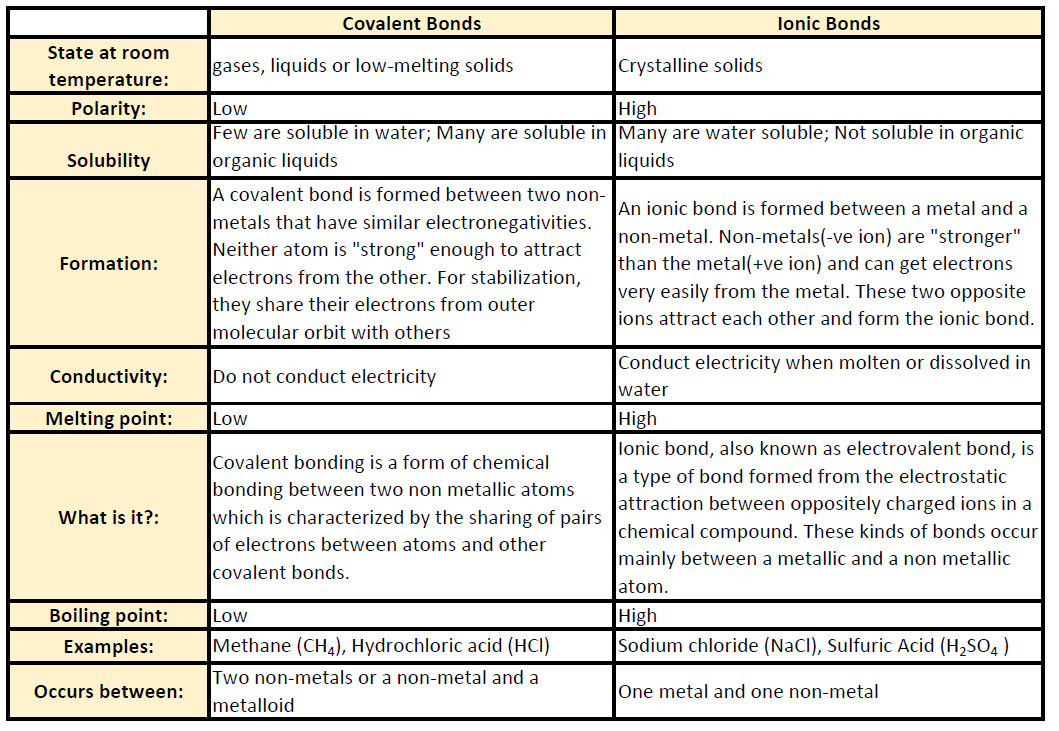
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
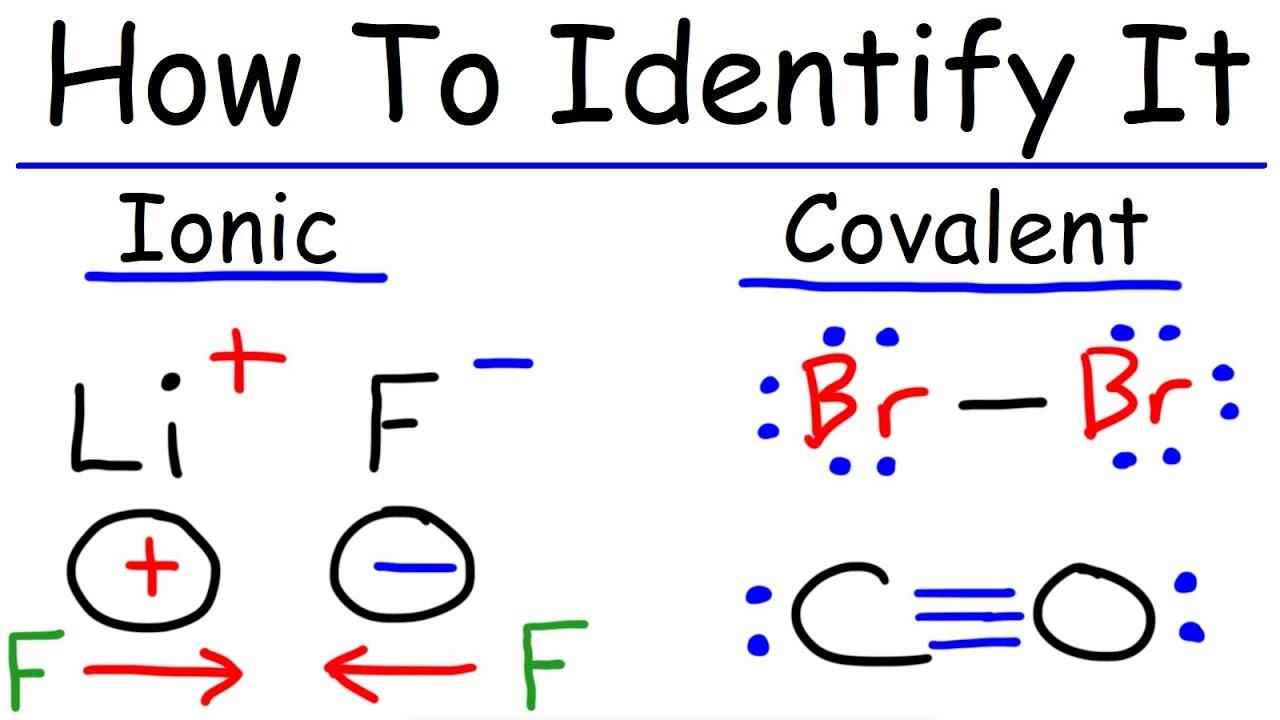
Ionic And Covalent Bonding Chemistry Youtube

4 2 Ionic And Molecular Compounds Chem 1114 Introduction To Chemistry

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Study Chemistry

Introduction To Ionic Bonding And Covalent Bonding Youtube

This Activity Is A Way To Practice Classifying Chemical Formulas As Either Ionic Or Covalent Bon Covalent Bonding Worksheet Covalent Bonding Teaching Chemistry
