Some Molecular Compounds Dissolve In Water To Form Ions Is
First it could be due to the equal sharing of electrons between the atoms. At an atomic level an ionic crystal is a regular structure with the cation and anion alternating with each other and forming a three-dimensional structure based.
Chemistry The Central Science Chapter 4 Section 1
Likewise the salts negative ions eg.
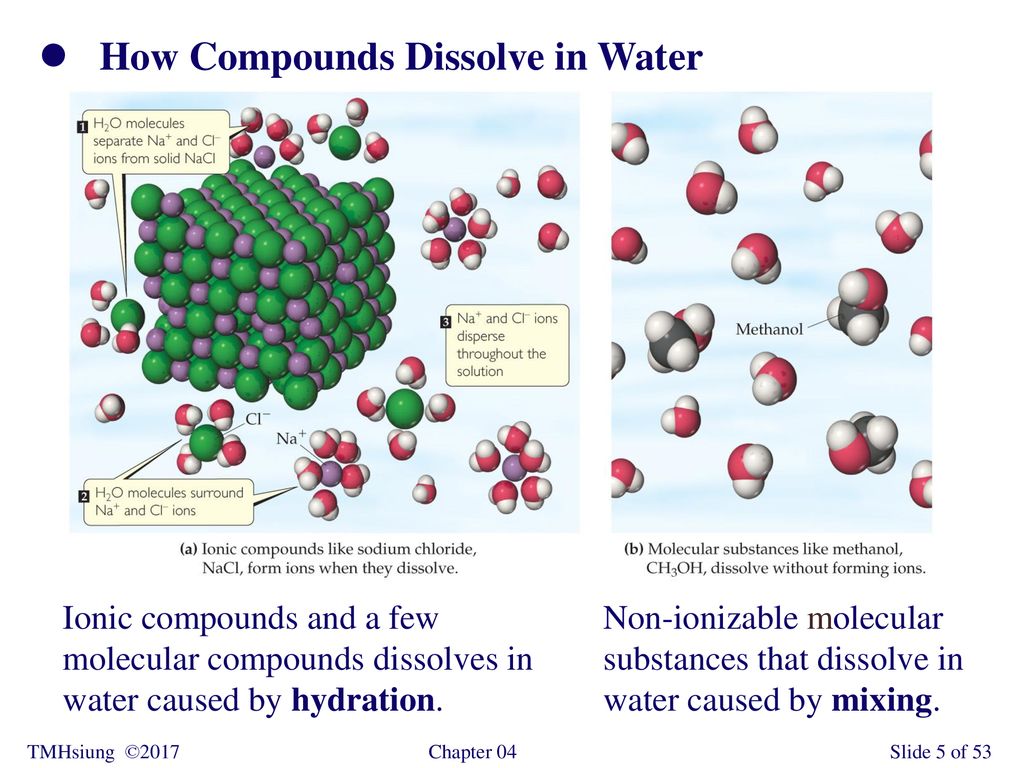
Some molecular compounds dissolve in water to form ions is. Oil and water do not mix. Metallic hydrides give hydrides ions. Calcium strontium and barium react with hydrogen to form metallic hydrides.
When soda ash is used to remove noncarbonate hardness an even higher pH is required - 100 to 105 for calcium compounds and 110 to 115 for magnesium compounds. Water molecules naturally disassociate into H and OH-ions which are pulled toward the cathode and anode respectively. Almost all the free oxygen in the atmosphere is due to photosynthesis.
Reaction of Alkaline Earth Metals with Water. Because these compounds are only slightly soluble assume that the volume does not change on dissolution and calculate the solubility product for each. A BaSiF 6 0026 g100 mL contains latextextSiF_62-latex ions.
The gases produced bubble to the surface where they can be collected. Hydrogen bonds are not readily formed with nonpolar substances like oils and fats. Ag attract the partially negative oxygens in H 2 O.
Or it may be heteronuclear a chemical compound composed of more than one element as with water two hydrogen atoms and one oxygen atom. About 3 parts of oxygen by volume dissolve in 100 parts of fresh water at 20 C 68 F. The solubility of a substance fundamentally depends on the solvent used as well as on temperature and pressure.
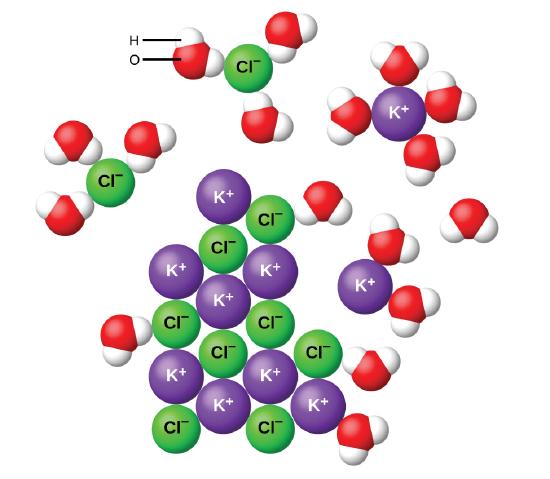
For example the salts positive ions eg. Figure 27 As this macroscopic image of oil and water shows oil is a nonpolar compound and hence will not dissolve in water. Hydrides react violently with water to release hydrogen.
These nonpolar compounds are hydrophobic water-fearing and will not dissolve in water. Calcium compounds in water will be removed at a pH of about 90 to 95 while magnesium compounds require a pH of 100 to 105. Secondly it could be due to the symmetrical arrangement of polar bonds into a more complex molecule such as the boron trifluoride BF 3An important fact that one needs to take note of is that not every molecule with polar bonds is a polar molecule.
Calcium hydride called Hydrolith is used for producing hydrogen. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a homogeneous solution of the solute in the solvent. Cl attract the partially positive hydrogens in H 2 O.
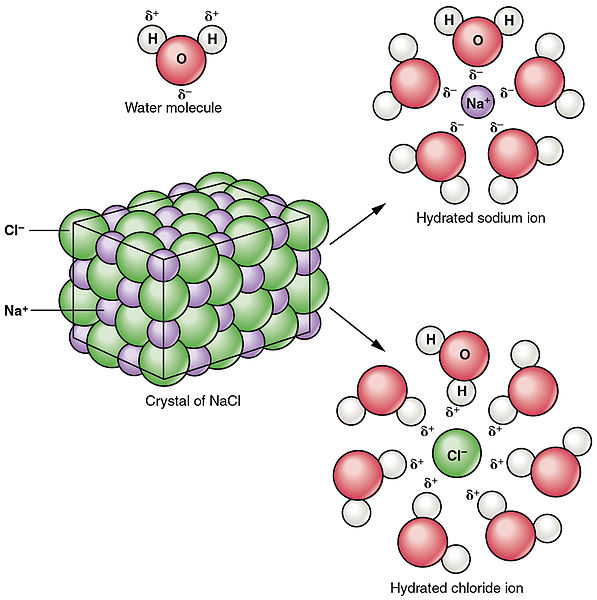
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. During respiration animals and some bacteria take oxygen from the atmosphere and return to it carbon dioxide whereas by photosynthesis green plants assimilate carbon dioxide in the presence of sunlight and evolve free oxygen. Some ionic compounds dissolve in water which arises because of the attraction between positive and negative charges see.
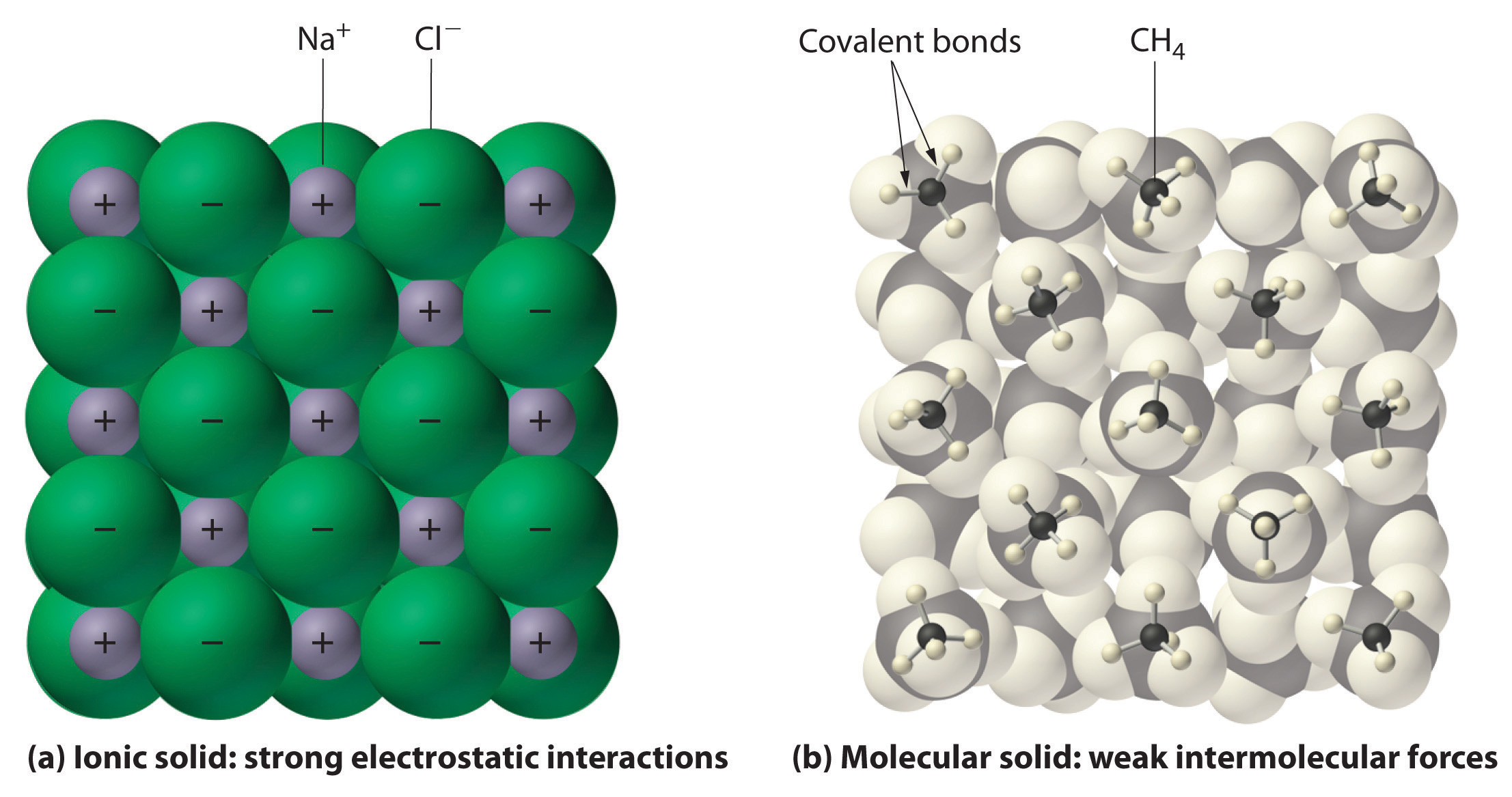
Unlike water the non-polar molecules arise in two cases. CaH 2 2H 2 O CaOH 2 H 2. Although molecular compounds form crystals they frequently take other forms plus molecular crystals typically are softer than ionic crystals.
At the anode four OH-ions combine and release O 2 gas molecular water and four electrons. At the cathode two H ions pick up electrons and form H 2 gas. They form crystals.
A molecule may be homonuclear that is it consists of atoms of one chemical element as with two atoms in the oxygen molecule O 2. M H 2 2MH 2 M 2 H. Ionic compounds form crystal lattices rather than amorphous solids.
The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water.
6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts

Chemistry 101 Chap 4 Aqueous Reactions And Solution Stoichiometry 1 General Properties Of Aqueous Solutions 2 Precipitation Reactions 3 Acid Base Ppt Download
What Determines Whether A Solid Is Soluble In Water Socratic

Chemistry Ii Water And Organic Molecules
What Happens When Ionic Compounds Dissolve In Water Quora

Dissolution In Water A When An Ionic Co Clutch Prep

Reactions In Aqueous Solution Ppt Download

General Solution Chemistry Molarity Properties Of Solutions 1
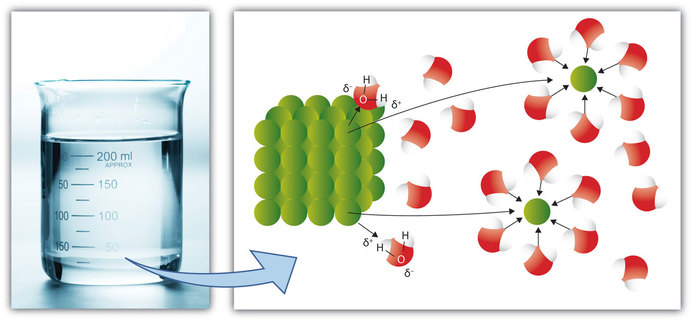
9 3 The Dissolution Process Chemistry Libretexts
Chemistry The Central Science Chapter 4 Section 1
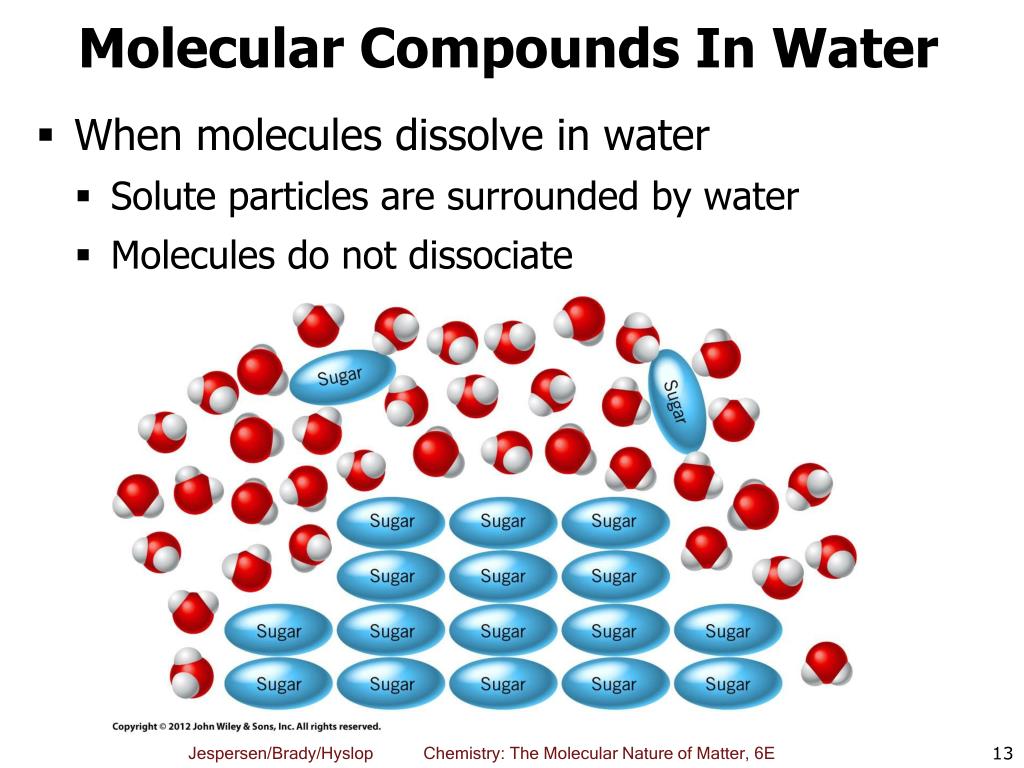
Ppt Chapter 5 Molecular View Of Reactions In Aqueous Solutions Part I Powerpoint Presentation Id 5074655

Chapter 4 Reactions In Aqueous Solution Part 1 Of 8 Youtube

Dissolving Process Chemistry For Non Majors
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora

Sort The Following Compounds Based On Whet Clutch Prep
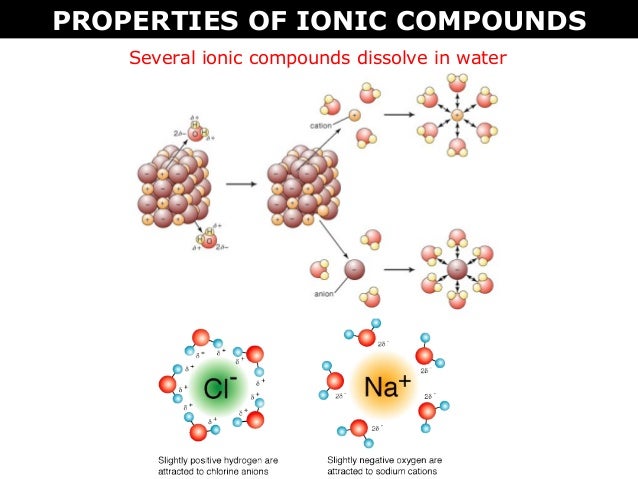
02 B Ionic Vs Molecular Compounds Bohr Rutherford And Lewis


